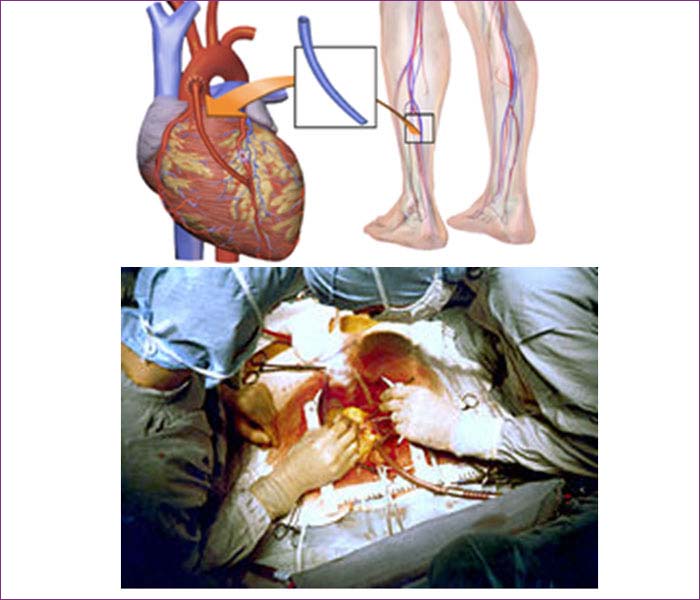
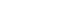Cardiac Surgery
Beating Heart Coronary Artery BypassGrafting (CABG).
Coronary artery bypass surgery, also known as coronary artery bypass graft (CABG, pronounced "cabbage") surgery, and colloquially heart bypass or bypass surgery, is a surgical procedure consisting of either diverting the left internal thoracic artery (left internal mammary artery or "LIMA") to the left anterior descending (LAD) branch of the left main coronary artery; or a harvested great saphenous vein of the leg, attaching the proximal end to the aorta or one of its major branches, and the distal end to immediately beyond a partially obstructed coronary artery (the "target vessel") - usually a 50% to 99% obstruction. The purpose is to restore normal blood flow to that partially obstructed coronary artery. It is performed to relieve angina unsatisfactorily controlled by maximum tolerated anti-ischemic medication, prevent or relieve left ventricular dysfunction, and/or reduce the risk of death. It does not prevent heart attacks. This surgery is usually performed with the heart stopped, necessitating the usage of cardiopulmonary bypass; however, two alternative techniques are also available allowing CABG to be performed on a beating heart either without using the cardiopulmonary bypass deemed as "off-pump" surgery or performing beating surgery using partial assistance of the cardiopulmonary bypass called as "on-pump beating" surgery. The latter gathers the advantages of the on-pump stopped and off-pump while minimizing their respective side-effects.
The obstruction being bypassed is due to arteriosclerosis, atherosclerosis, or both. Arteriosclerosis is characterized by thickening, loss of elasticity, and calcification of the arterial wall, most often resulting in a generalized narrowing in the affected coronary artery. Atherosclerosis is characterized by yellowish plaques of cholesterol, lipids, and cellular debris deposited into the inner layer of the wall of a large or medium-sized coronary artery, most often resulting in a focal partial obstruction in the affected artery, each can limit blood flow if it causes a cross-sectional narrowing of at least 50%.

Benefits
A safer and more permanent and successful way to prevent heart attacks in patients at high risk is to exercise, give up smoking, take "drugs to get blood pressure under control and drive cholesterol levels down to prevent blood clotting". Longer term, behavioral and medication treatment may be the only way to avoid vascular related loss of mental function.
Procedure
Valve Replacement / Repairs
1. Mitral Valve
Mitral valve replacement is a cardiac surgical procedure in which a patient’s diseased mitral valve is replaced by either a mechanical or bio prosthetic valve. Mitral valve replacement is performed when the valve becomes too tight (mitral valve stenosis) for blood to flow into the leftventricle, or too loose (mitral valve regurgitation) in which case blood can leak back into the left atrium and thereby back into the lung. Mitral valve disease can occur from infection, calcification, inherited collagen disease, or other causes. Since a mitral valve replacement is anopen heart surgical procedure, it requires placing the patient on cardiopulmonary bypass.
Many mitral valves can be repaired instead of replaced, especially for minimally damaged valves. Advantages to valve repair instead of replacement include lower surgical mortality (1-2% for repair versus 6-8% for replacement), lower risk of stroke, lower rate of endocardial infection, and improved long-term survival. Patients who receive a valve repair stay on the same survival curve as the normal population. After mitral valve repair, blood thinners are not required; however, lifelong maintenance on blood thinners is required after mechanical mitral valve replacement. Mitral valve surgery can now also be performed robotically although the procedure may take longer.
Types of Valves
There are two primary types of artificial mitral valves: mechanical valves and bioprosthetic tissue (biological) valves. The mechanical valves are made from metal and pyrolytic carbon, and can last a lifetime. Patients with mechanical valves must take blood-thinning medications to prevent clotting. Bioprosthetic valves are made from animal tissues. Use of these biological valves allows patients to avoid blood thinners. However, the bioprosthetic valves may only last 10 to 15 years.[6] The choice of which valve type to use depends upon the patient's age, medical condition, preferences with medication, and lifestyle.
Details of the procedure
Patients having mitral valve surgery receive general anesthesia. Incision can be made somewhat horizontally under the left breast, or vertically through the sternum. After the heart is exposed, canulae are placed to rerout blood to a heart-lung machine for cardiopulmonary bypass. An incision is made in the left atrium to expose the mitral valve. The valve is then replaced with either a biological or mechanical valve. The left artium is then closed, and the patient weaned from cardiopulmonary bypass. After surgery patients are typically taken to an intensive care unit (ICU).
2. Aortic valve
The aortic valve is one of the two semilunar valves of the heart, the other being the pulmonary valve. The heart has four valves and the other two are the mitral and the tricuspid valves. The aortic valve normally has three cusps or leaflets, although in 1-2% of the population it is found to congenitally have two leaflets. It lies between the left ventricle and the aorta.
Aortic valve replacement is a surgical procedure in which a patient's aortic valve is replaced by a different valve. The aortic valve can be affected by a range of diseases and require aortic valve replacement. The valve can become either leaky (regurgitant or insufficient) or stuck partially shut (stenotic). Aortic valve replacement currently requires open heart surgery. Research is being done now to develop valves that can be implanted using a catheter without open heart surgery. There are two basic types of artificial heart valve, mechanical valves and tissue valves. Tissue heart valves are usually made from animal tissues, either animal heart valve tissue or animal pericardial tissue. The tissue is treated to prevent rejection and to prevent calcification.
There are alternatives to animal tissue valves. In some cases, a human aortic valve can be implanted. These are called homografts. Homograft valves are donated by patients and recovered after the patient expires. The durability of homograft valves is probably the same as for porcine tissue valves. Another procedure for aortic valve replacement is the Ross procedure (after Donald Ross) or pulmonary autograft. The Ross procedure involves going to surgery to have the aortic valve removed and replacing it with the patient's own pulmonary valve. A pulmonary homograft (a pulmonary valve taken from a cadaver) or a valvular prothesis is then used to replace the patient's own pulmonary valve.
Complications of Myocardial Infarction ‐Ventricular septal rupture.
Myocardial infarction (MI) or acute myocardial infarction (AMI), commonly known as a heart attack, occurs when blood flow stops to part of the heart causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may travel into the shoulder, arm, back, neck, or jaw. Often it is in the center or left side of the chest and lasts for more than a few minutes. The discomfort may occasionally feel like heartburn. Other symptoms may include shortness of breath, nausea, feeling faint, a cold sweat, or feeling tired. About 30% of people have atypical symptoms, with women more likely than men to present atypically. Among those over 75 years old, about 5% have had an MI with little or no history of symptoms. An MI may cause heart failure, an irregular heartbeat, or cardiac arrest.
Most MIs occur due to coronary artery disease. Risk factors include high blood pressure, smoking, diabetes, lack of exercise, obesity, high blood cholesterol, poor diet, and excessive alcohol, among others. The mechanism of an MI often involves the rupture of an atherosclerotic plaque leading to complete blockage of a coronary artery. MIs are less commonly caused by coronary artery spasms which may be due tococaine, significant emotional stress, and extreme cold, among others. A number of tests are useful to help with diagnosis includingelectrocardiograms (ECGs), blood tests, and coronary angiography. An ECG may confirm an ST elevation MI if ST elevation is present.Commonly used blood tests include troponin and less often Creatine.
Signs and symptoms
The onset of symptoms in myocardial infarction (MI) is usually gradual, over several minutes, and rarely instantaneous. Chest pain is the most common symptom of acute MI and is often described as a sensation of tightness, pressure, or squeezing. Chest pain due to ischemia (a lack of blood and hence oxygen supply) of the heart muscle is termed angina pectoris. Pain radiates most often to the left arm, but may also radiate to the lower jaw, neck, right arm, back, and upper abdomen, where it may mimic heartburn. Levine's sign, in which a person localizes the chest pain by clenching their fists over their sternum, has classically been thought to be predictive of cardiac chest pain, although a prospective observational study showed it had a poor positive predictive value.
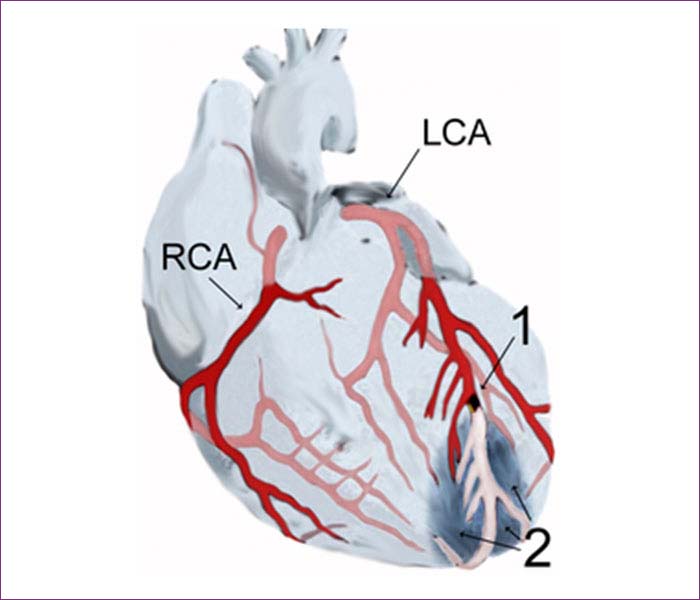
Diagram of a myocardial infarction (2) of the anterior wall of the heart after blockage (1) of a branch of the left coronary artery (LCA). In the diagram, RCA is the right coronary artery.
Shortness of breath (dyspnea) occurs when the damage to the heart limits the output of the left ventricle, causing left ventricular failure and consequent pulmonary edema. Other symptoms include diaphoresis (an excessive form of sweating), weakness, light-headedness, nausea, vomiting, and palpitations. These symptoms are likely induced by a massive surge of catecholamines from the sympathetic nervous system, which occurs in response to pain and the blood flow abnormalities that result from dysfunction of the heart muscle. Loss of consciousness (due to inadequate blood flow to the brain and cardiogenic shock) and sudden death (frequently due to the development of ventricular fibrillation) can occur in MIs.
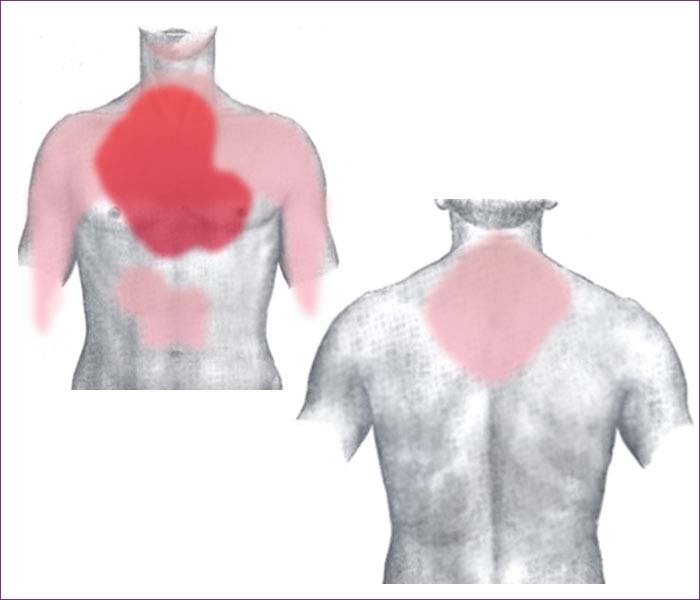
Rough diagram of pain zones in myocardial infarction; dark red: most typical area, light red: other possible areas; view of the chest& Back view Atypical symptoms are more frequently reported by women, the elderly, and those with diabetes when compared to their male and younger counterparts. Women also report more numerous symptoms compared with men (2.6 on average vs. 1.8 symptoms in men). The most common symptoms of MI in women include dyspnea, weakness, and fatigue. Fatigue, sleep disturbances, and dyspnea have been reported as frequently occurring symptoms that may manifest as long as one month before the actual clinically manifested ischemic event. In women, chest pain may be less predictive of coronary ischemia than in men. Women may also experience back or jaw pain during an episode.
At least one quarter of all MIs are silent, without chest pain or other symptoms. These cases can be discovered later on electrocardiograms, using blood enzyme tests, or at autopsy without a prior history of related complaints. Estimates of the prevalence of silent MIs vary between 22 and 64%.A silent course is more common in the elderly, in people with diabetes mellitus and after heart transplantation, probably because the donor heart is not fully innervated by the nervous system of the recipient. In people with diabetes, differences in pain threshold, autonomic neuropathy, andpsychological factors have been cited as possible explanations for the lack of symptoms.
Any group of symptoms compatible with a sudden interruption of the blood flow to the heart, which includes STEMI, NSTEMI or unstable angina, are called an acute coronary syndrome.
Complications
Complications may occur immediately following the heart attack (in the acute phase), or may need time to develop (a chronic problem). Acute complications may include heart failure if the damaged heart is no longer able to pump blood adequately around the body; aneurysm of the left ventricle myocardium; ventricular septal rupture or free wall rupture; mitral regurgitation, in particular if the infarction causes dysfunction of the papillary muscle; Dressler's syndrome; and abnormal heart rhythms, such as ventricular fibrillation, ventricular tachycardia, atrial fibrillation, and heart block. Longer-term complications include heart failure, atrial fibrillation, and an increased risk of a second MI.
Ventricular Septal Rupture
Ventricular septal rupture (VSR) is a rare but lethal complication of myocardial infarction (MI). The event occurs 2-8 days after an infarction and often precipitates cardiogenic.The differential diagnosis of post infarction cardiogenic shock should exclude free ventricular wall rupture and rupture of the papillary muscles. (See the image below.)
To avoid the high morbidity and mortality associated with this disorder, patients should undergo emergency surgical treatmentIn current practice, postinfarction VSR is recognized as a surgical emergency, and the presence of cardiogenic shock is an indication for intervention.Long-term survival can be achieved in patients who undergo prompt surgery. Concomitant coronary artery bypass grafting (CABG) may be required. The addition of CABG has helped improve long-term survival.
Surgery is performed via a transinfarction approach, and all reconstruction is performed with prosthetic materials to avoid tension. Developments in myocardial protection and improved prosthetic materials have contributed greatly to successful management of VSR.Improved surgical techniques (eg, infarctectomy) and better perioperative mechanical and pharmacologic support have helped lower mortality. In addition, the development of surgical techniques to repair perforations in different areas of the septum has led to improved results.
In current practice, patients undergoing shunt repair tend to be older and are more likely to have received thrombolytic agents, which may complicate repair. After successful repair, survival and quality of life are excellent, even in patients older than 70 years.
Congenital Heart Diseases.
1. Arial Septal Defects Ventricular Septa Defects
An atrial septal defect (ASD) — sometimes referred to as a hole in the heart — is a type of congenital heart defect in which there is an abnormal opening in the dividing wall between the upper filling chambers of the heart (the atria). In most cases ASDs are diagnosed and treated successfully with few or no complications.
To understand this defect, it first helps to review some basics about the way a healthy heart typically works.
The heart has four chambers: The two lower pumping chambers are called the ventricles, and the two upper filling chambers are the atria.
In a healthy heart, blood that returns from the body to the right-sided filling chamber (right atrium) is low in oxygen. This blood passes to the right-sided pumping chamber (right ventricle), and then to the lungs to receive oxygen. The blood that has been enriched with oxygen returns to the left atrium, and then to the left ventricle. It's then pumped out to the body through the aorta, a large blood vessel that carries the blood to the smaller blood vessels in the body. The right and left filling chambers are separated by a thin shared wall, called the atrial septum.
Kids with an atrial septal defect have an opening in the wall (septum) between the atria. As a result, some oxygenated blood from the left atrium flows through the hole in the septum into the right atrium, where it mixes with oxygen-poor blood and increases the total amount of blood that flows toward the lungs. The increased blood flow to the lungs creates a swishing sound, known as a heart murmur. This heart murmur, along with other specific heart sounds that can be detected by a cardiologist, can be clues that a child has an ASD.
ASDs can be located in different places on the atrial septum, and they can be different sizes. The symptoms and medical treatment of the defect will depend on those factors. In some rare cases, ASDs are part of more complex types of congenital heart disease. It's not clear why, but ASDs are more common in girls than in boys.
Causes
ASDs occur during fetal development of the heart and are present at birth. During the first weeks afterconception, the heart develops. If a problem occurs during this process, a hole in the atrial septum may result.
In some cases, the tendency to develop a ASD might be genetic. Genetic syndromes can cause extra or missing pieces of chromosomes that can be associated with ASD. For the vast majority of children with a defect, however, there's no clear cause.
Signs and Symptoms
The size of an ASD and its location in the heart will determine what kinds of symptoms a childexperiences. Most kids who have ASDs seem healthy and appear to have no symptoms. Generally, they feel well and grow and gain weight normally.
Children with larger, more severe ASDs, however, might have some of these signs or symptoms:
If an ASD is not treated, health complications can develop later, including an abnormal heart rhythm (known as an atrial arrhythmia) and problems with how well the heart pumps blood. As kids with ASDs get older, they also might be at an increased risk for stroke, since a blood clot that develops can pass through the hole in the wall between the atria and travel to the brain. Pulmonary hypertension (high blood pressure in the lungs) also can develop over time in older patients with larger untreated ASDs.
Fortunately, most kids with ASD are diagnosed and treated long before the heart defect causes physical symptoms. Because of the complications that ASDs can cause later in life, pediatric cardiologists often recommend closing ASDs early in childhood.
Diagnosis
Generally, a child's doctor hears the heart murmur caused by ASD during a routine checkup or physical examination. ASDs are not always diagnosed as early in life as other types of heart problems, such as ventricular septal defect (a hole in the wall between the two ventricles). The murmur caused by an ASD is not as loud and can be harder to hear than other types of heart murmurs, so it may be diagnosed any time between infancy and adolescence (or even as late as adulthood).
If a doctor hears a murmur and suspects a heart defect, the child may be referred to a pediatric cardiologist (a doctor who specializes in diagnosing and treating childhood heart conditions). If an ASD is suspected, the cardiologist might order one or more of the following tests:
Treatment
Once an ASD is diagnosed, treatment will depend on the child's age and the size, location, and severity of the defect. In kids with very small ASDs, the defect may close on its own. Larger ASDs usually won't close, and must be treated medically. Most of these can be closed in a cardiac catheterization lab, although some will require open-heart surgery.
A child with a small defect that causes no symptoms may simply need to visit a pediatric cardiologist regularly to ensure that there are no problems; often, small defects will close spontaneously without any treatment during the first years of life. In general, kids with a small ASD won't require restrictions on physical activity.
In most children with ASD, though, doctors must close the defect if it has not closed on its own by the time a child is old enough to start school.
Depending on the defect's position, many can be corrected by cardiac catheterization. In this procedure, a catheter (a thin, flexible tube) is inserted into a blood vessel in the leg that leads to the heart. A cardiologist guides the tube into the heart to make measurements of blood flow, pressure, and oxygen levels in the heart chambers. A special implant is positioned into the hole in the septum and will flatten against the septum on both sides to close and permanently seal the ASD.
In the beginning, the natural pressure in the heart holds the device in place. Over time, the normal tissue of the heart grows over the device and covers it entirely. This non-surgical technique for closing an ASD eliminates the scar on the chest needed for the surgical approach, and has a shorter recovery time, usually just an overnight stay in the hospital.
There's a small risk of blood clots forming on the closure device while new tissue heals over it, so kids who have their ASD closed with a device implantation are prescribed a low dose of aspirin for 6 months after the procedure.
If surgical repair for ASD is necessary, a child will undergo open-heart surgery. In this procedure, a surgeon makes a cut in the chest and a heart-lung machine is used to maintain circulation while the heart surgeon closes the hole. The ASD may be closed directly with stitches or by sewing a patch of surgical material over the defect. Eventually, the tissue of the heart heals over the patch or stitches, and by 6 months after the surgery, the hole will be completely covered with tissue.
For 6 months following catheterization or surgical closure of an ASD, antibiotics are recommended before routine dental work or surgical procedures to prevent infective endocarditis (an infection of the inner surface of the heart). Once the heart tissue has healed over the closed ASD, most patients no longer need to worry about having a higher risk of infective endocarditis.
Your doctor will discuss other possible risks and complications with you prior to the procedure. Typically, after repair and adequate time for healing, kids with ASD rarely have further symptoms.
Caring for Your Child
Kids who undergo cardiac catheterization to close an ASD usually spend the night in the hospital after the procedure and also should be kept out of gym class or sports practice for a week. After that, they can usually return to their normal physical activities, with their doctor's OK.
Kids who have surgery for their ASDs usually go home after a few days in the hospital if there are no complications. After surgical ASD repair, the main medical concern is the healing of the chest incision. In general, the younger patients are when they have their surgical repairs, the less pain they will have during recovery. The child will be watched closely for signs or symptoms that may indicate a problem. If your child has trouble breathing, is not eating, has fever, or redness or pus oozing from the incision, get medical treatment right away. In most cases, kids who have had ASD surgery recover quickly and without problems.
In the weeks following surgery or cardiac catheterization, your doctor will check on your child's progress. Your child may undergo another echocardiogram to make sure that the heart defect has closed completely. Kids who have undergone ASD repair will continue to have follow-up visits with the cardiologist.
Most kids recover from treatment quickly — you might even notice that within a few weeks, your child is eating more and is more active than before surgery. However, some signs and symptoms might indicate a problem. If your child is having trouble breathing, call the doctor or go to the emergency department immediately.
Other symptoms that can indicate a problem include:
Any time a child is diagnosed with a heart condition, it can be scary. But the good news is that your pediatric cardiologist will be very familiar with this condition and how to best manage it. Most kids who've had an ASD corrected have a normal life expectancy and go on to live healthy, active lives.
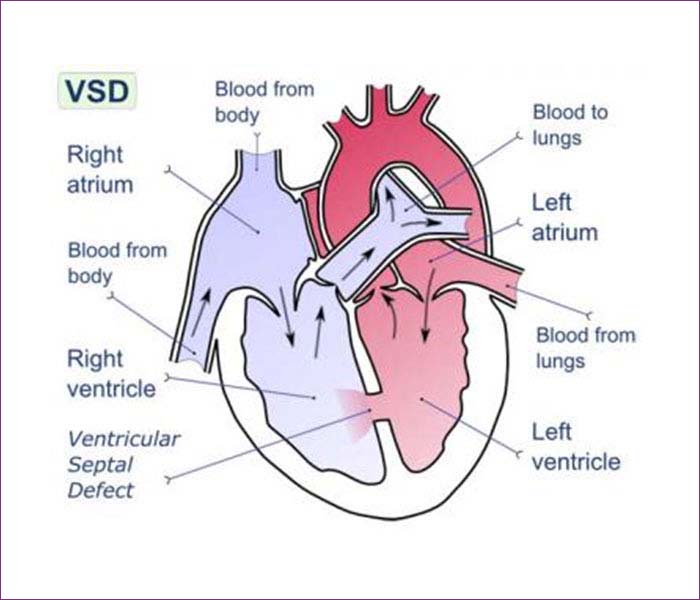
2. Ventricular Septal Defect
A ventricular septal defect (VSD) — sometimes referred to as a hole in the heart — is a type of congenital heart defect in which there is an abnormal opening in the dividing wall between the main pumping chambers of the heart (the ventricles).
VSDs are the most common congenital heart defect, and in most cases they're diagnosed and treated successfully with few or no complications.
What Is a Ventricular Septal Defect?
To understand this defect, it first helps to review some basics about the way a healthy heart typically works.
The heart has four chambers: The two lower pumping chambers of the heart are called the ventricles, and the two upper filling chambers are the atria.
In a healthy heart, blood that returns from the body to the right-sided filling chamber (right atrium) is low in oxygen. This blood passes to the right-sided pumping chamber (right ventricle), and then travels to the lungs to receive oxygen. The blood that has been enriched with oxygen returns to the left atrium, and then to the left ventricle. It's then pumped out to the body through the aorta, a large blood vessel that carries the blood to the smaller blood vessels in the body.
The right and left-sided pumping chambers (ventricles) are separated by shared wall, called the ventricular septum.
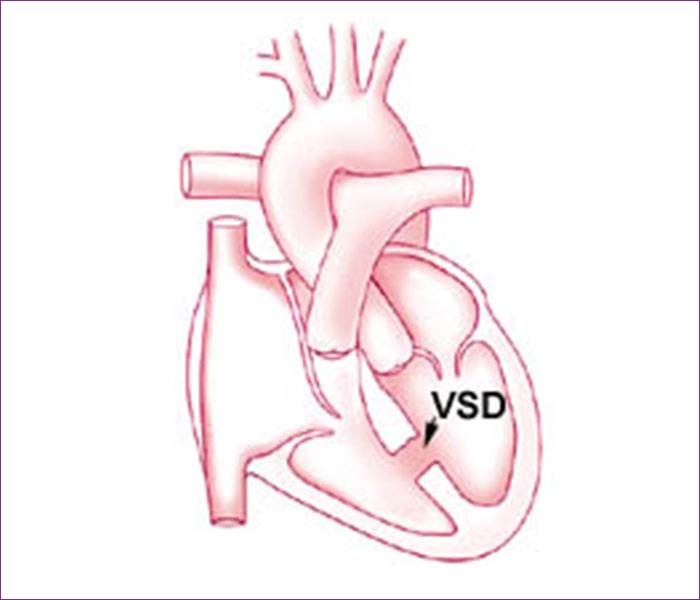
Kids with a VSD have an opening in this wall. As a result, when the heart beats, some of the blood in the left ventricle (which has been enriched by oxygen from the lungs) is able to flow through the hole in the septum into the right ventricle. In the right ventricle, this oxygen-rich blood mixes with the oxygen-poor blood and goes back to the lungs. The blood flowing through the hole creates an extra noise, which is known as a heart murmur. The heart murmur can be heard when a doctor listens to the heart beat with a stethoscope.
VSDs can be located in different places on the ventricular septum, and they can be different sizes. The symptoms and medical treatment of the VSD will depend on those factors. In some rare cases, VSDs are part of more complex types of congenital heart disease.
What Causes a VSD?
Ventricular septal defects occur during fetal heart development and are present at birth. During the first weeks after conception, the heart develops from a large tube, dividing into sections that will eventually become the walls and chambers. If a problem occurs during this process, it can create a hole in the ventricular septum.
In some cases, the tendency to develop a VSD may be due to genetic syndromes that cause extra or missing pieces of chromosomes. The vast majority of these defects, though, have no clear cause.
Signs and Symptoms
VSDs are usually found in the first few weeks of life by a doctor during a routine checkup. The doctor will be able to detect a heart murmur, which is due to the sound of blood as it passes between the left and right ventricles. The murmur associated with a VSD has certain features that allow a doctor to distinguish it from heart murmurs due to other causes.
The size of the hole and its location within the heart will determine whether a VSD causes any symptoms. Small VSDs will not typically cause any symptoms, and may ultimately close on their own. Older kids or teens who have small VSDs that persist usually don't experience any symptoms other than the heart murmur that doctors hear. They might need to see a doctor regularly to check on the heart defect and make sure it isn't causing any problems.
Moderate and large VSDs that haven't been treated in childhood may cause noticeable symptoms. Babies may have faster breathing and get tired out during attempts to feed. They may start sweating or crying with feeding, and may gain weight at a slower rate.
These signs generally indicate that the VSD will not close by itself, and cardiac surgery may be needed. This usually is done within the first 3 months of life to prevent other complications. A cardiologist can prescribe medication to lessen symptoms before surgery.
People with a VSD are at greater risk in their lifetime of developingendocarditis, an infection of the inner surface of the heart. This occurs when bacteria in the bloodstream infect the lining of the heart. Bacteria are always in our mouths, and small amounts are introduced into the bloodstream when we chew and brush our teeth. The best way to protect the heart from endocarditis is to reduce oral bacteria by brushing and flossing daily, and visiting the dentist regularly. In general, it is not recommended that patients with simple VSDs take antibiotics before dental visits, except for the first 6 months after surgery.
Diagnosing a VSD
If your child is discovered to have a heart murmur, a doctor may refer you to a pediatric cardiologist, a doctor who specializes in diagnosing and treating childhood heart conditions.
In addition to doing a physical exam, the cardiologist takes your child's medical history. If a VSD is suspected, the cardiologist may order one or more of these tests:
Treating a VSD
Once an VSD is diagnosed, treatment will depend on the child's age and the size, location, and severity of the defect. A child with a small defect that causes no symptoms may simply need to visit a cardiologist regularly to make sure that there are no other problems.
In most kids, a small defect will close on its own without surgery. Some might not close but do not get any larger. Kids whose VSD is small and has not closed generally won't have to restrict their physical activities.
For kids with medium to large VSDs, surgery may be necessary. In most cases, this takes place within the first few weeks to months of life. In this procedure, the surgeon makes an incision in the chest wall and a heart-lung machine will maintain circulation while the surgeon closes the hole. The surgeon can stitch the hole closed directly or, more commonly, sew a patch of manmade surgical material over it. Eventually, the tissue of the heart heals over the patch or stitches, and by 6 months after the surgery, the hole will be completely covered with tissue.
Certain types of VSDs may be closed by cardiac catheterization. A thin, flexible tube (a catheter) is inserted into a blood vessel in the leg that leads to the heart. A cardiologist guides the tube into the heart to make measurements of blood flow, pressure, and oxygen levels in the heart chambers. A special implant, shaped into two disks formed of flexible wire mesh, is positioned into the hole in the septum. The device is designed to flatten against the septum on both sides to close and permanently seal the VSD.
After healing from an operation to repair the defect, a child with a VSD should have no further symptoms or problems.
Caring for a Child with a VSD
Some kids with VSDs may take heart medication prior to surgery to help lessen the symptoms from the defect. Those who have surgery for larger VSDs usually leave the hospital within 4 to 5 days after surgery if there are no problems.
In most cases, kids who have had VSD surgery recover quickly and without problems. But doctors will closely monitor the child for signs or symptoms of any problems.
Your child may undergo another echocardiogram to make sure that the heart defect has closed completely. If your child is having trouble breathing, call your doctor or go to the emergency department immediately.
Other symptoms that may indicate a problem include:
Call your doctor if you notice any of these signs in your child after closure of the VSD.
Any time a child is diagnosed with a heart condition, it can be scary. But the good news is that your pediatric cardiologist will be very familiar with this condition and how to best manage it. Most kids who've had a VSD corrected have a normal life expectancy and go on to live healthy, active lives.
3. Patent Ductus Arteriosus (PDA)
The ductus arteriosus (DA) is a normal blood vessel that connects two major arteries — the aorta and the pulmonary artery — that carry blood away from the heart in a developing fetus. The DA diverts blood away from the lungs, sending it directly to the body.
The lungs are not used while a fetus is in the amniotic fluid because the baby gets oxygen directly from the mother's placenta. When a newborn breathes and begins to use the lungs, the DA is no longer needed and usually closes during the first 2 days after birth.
But when the DA fails to close, a condition called patent (meaning "open") ductus arteriosus (PDA) results, in which oxygen-rich blood from the aorta is allowed to mix with oxygen-poor blood in the pulmonary artery. As a result, too much blood flows into the lungs, which puts a strain on the heart and increases blood pressure in the pulmonary arteries.
Causes
The cause of PDA is not known, but genetics might play a role. PDA is more common in premature babies and affects twice as many girls as boys. It's also common among babies with neonatal respiratory distress syndrome, babies with genetic disorders (such as Down syndrome), and babies whose mothers had German measles (rubella) during pregnancy.
In the vast majority of babies with a PDA but an otherwise normal heart, the PDA will shrink and go away on its own in the first few days of life. Some PDAs that don't close then will close on their own by the time the child is a year old.
In premature infants, the PDA is more likely to stay open, particularly if the baby has lung disease. When this happens, treatment to close the PDA might be considered.
In infants born with additional heart defects that decrease blood flow from the heart to the lungs or decrease the flow of oxygen-rich blood to the body, the PDA could actually be beneficial and the doctor might prescribe medicine to keep the ductus arteriosus open.
Symptoms and Tests
Babies with a large PDA might experience symptoms such as:
If a PDA is suspected, the doctor will use a stethoscope to listen for a heart murmur, which is often heard in babies with PDAs. Follow-up tests might include:
Treatment
The three treatment options for PDA are medication, catheter-based procedures, and surgery. A doctor will close a PDA if the size of the opening is large enough that the lungs could become overloaded with blood, a condition that can lead to an enlarged heart.
A PDA also might be closed to reduce the risk of developing a heart infection known as endocarditis, which affects the tissue lining the heart and blood vessels. Endocarditis is serious and requires treatment with intravenous (IV) antibiotics.
4. AtrioventricularSeptal Defect − AV Canal
Atrioventricular septal defects (AVSD) are a relatively common family of congenital heart defects. Also known as atrioventricular canal defects or endocardial cushion defects, they account for about 5 percent of all congenital heart disease, and are most common in infants with Down syndrome. (About 15 percent to 20 percent of newborns with Down syndrome have atrioventricular septal defects).
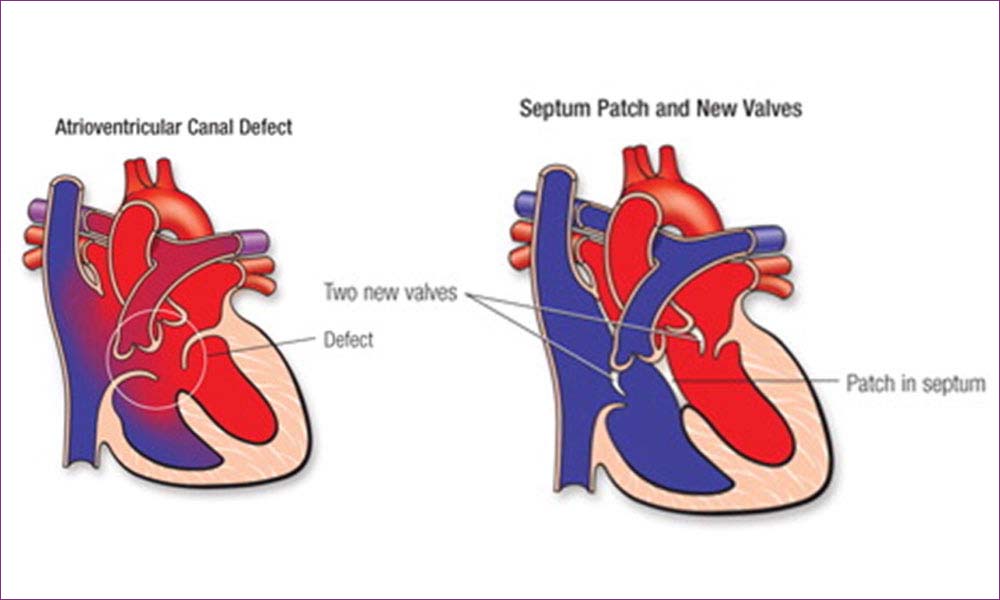
The primary defect is the failure of formation of the part of the heart that arises from an embryonic structure called the endocardial cushions. The endocardial cushions are responsible for separating the central parts of the heart near the tricuspid and mitral valves (AV valves), which separate the atria from the ventricles.
The structures that develop from the endocardial cushions include the lower part of the atrial septum (wall that divides the right atrium from the left atrium) and the ventricular septum (wall that divides the right ventricle from the left ventricle) just below the tricuspid and mitral valves.
The endocardial cushions also complete the separation of the mitral and tricuspid valves by dividing the single valve between the embryonic atria and ventricles. An atrioventricular septal defect may involve failure of formation of any or all of these structures.
Signs & Symptoms
Babies with congestive heart failure breathe fast and hard, often sweat and / or tire out while feeding, and grow slowly or sometimes even lose weight. These symptoms usually develop gradually over the first one to two months of life.
The doctor will usually hear a heart murmur when this type of defect is present. The murmur is caused by the blood passing from the left ventricle to the right ventricle and out the pulmonary artery.
A small number of infants with a complete atrioventricular septal defect will not develop congestive heart failure. This occurs because in some cases, the muscle cells that line the small arteries to the lungs get bigger and constrict to try to protect the lungs from the extra flow and high pressure caused by the atrioventricular septal defect.
Called increased pulmonary vascular resistance (PVR) or pulmonary vascular disease, this condition is more common in infants with Down syndrome.
The increase in pulmonary vascular resistance is very effective in preventing the signs and symptoms of congestive heart failure by minimizing the amount of left-to-right shunt, and may even cause blood with low oxygen to go from the right ventricle to the left ventricle and out to the body without picking up oxygen.
This causes cyanosis, which is a bluish discoloration of the skin, fingernails and mouth and it may also cause the murmur to be softer.
While infants with a complete atrioventricular septal defect and elevated pulmonary vascular resistance often grow better and appear healthier than those with low pulmonary vascular resistance and congestive heart failure, the occurrence of increased pulmonary vascular resistance may prompt early surgical correction of the defect.
Repair of the atrioventricular septal defect lowers the pressure in the pulmonary artery and allows these muscles to relax before they become permanently constricted.
Infants with the partial or transitional forms of atrioventricular septal defects have more subtle signs and symptoms. Like children with a complete atrioventricular septal defect, they have an increased volume of blood passing through the pulmonary artery.
The main difference between a left-to-right shunt that occurs primarily between the atria rather than the ventricles is that the pressure in the pulmonary artery usually remains low despite the increase in flow.
This causes less work for the heart and lungs and results in fewer breathing and growth problems. It also lessens the possibility that the pulmonary vascular resistance will increase.
Nevertheless, there is an increased workload on the heart and growth may occur more slowly than infants and children with normal hearts. There is usually a heart murmur present, but it is softer than that which occurs with a complete atrioventricular septal defect.
These types of defects may not come to medical attention until the child is several months or even years old because of the subtlety of the signs and symptoms that may be associated with them.
Significant congestive heart failure, growth failure or a very loud murmur in a child with a partial atrioventricular septal defect can occur when the defect in the mitral valve leaflet causes this valve to be very leaky.
A heart murmur is often the first clue that this heart defect exists. It is typically noted in the first week or two of life and it is not uncommon that no murmur is present at birth.
Diagnosis
The diagnosis of atrioventricular septal defect in any form is easily made by echocardiography. Chest X-ray and an electrocardiogram may be used to assist in the assessment. Both may show characteristic findings in atrioventricular septal defects.
Because of the high incidence of atrioventricular septal defects in infants with Down syndrome, all infants with Down syndrome should have an echocardiogram, even if they do not have a heart murmur or any of the signs or symptoms discussed above.
Symptomatic infants with atrioventricular septal defects may improve with medicine, but in all cases corrective heart surgery will be necessary.
These type of defects will never close on their own and will always require corrective surgery for treatment.
Medical treatment of infants with atrioventricular septal defects is usually used to relieve symptoms and allow the baby to get big enough to undergo surgical repair with lower risks.
This usually occurs at 3-6 months for infants with a complete atrioventricular septal defect and 6-18 months for infants with a partial atrioventricular septal defect.
Surgical repair of either type of defect involves closure of the holes in the atrial and / or ventricular septa with a patch or patches, and reconstruction of the common atrioventricular valve.
A particularly challenging aspect of the repair of a complete atrioventricular septal defect is dividing the common AV valve found in this condition.
Complications following surgery can arise if the opening in the mitral valve is now too narrow or it is still very leaky. Other problems to be avoided include narrowing the path for blood to pass from the left ventricle to the aorta, or disturbances of the electrical system of the heart.
The specialized tissue that conducts the impulse for the heart to beat runs very near the area where the stitches for the ventricular patch need to be placed. If this is disrupted, placement of a pacemaker may be necessary.
Atrioventricular Septal Defects Treatment Outcomes
The usual recovery period following repair of a partial atrioventricular septal defect is relatively brief. Most patients are out of the intensive care unit (ICU) in one to two days and home in four to five days following surgery.
Reported surgical survival is greater than 97 percent but is probably close to 100 percent in the current era.
Repair of a complete atrioventricular septal defect is often more complex and may be associated with other factors that can prolong the postoperative course.
In particular, the presence of elevated PVR preoperatively can necessitate a prolonged time on a mechanical ventilator and the need for higher amounts of medication to help the heart work well after surgery.
Additionally, problems with the mitral valve being too leaky, the path out of the left ventricle being too narrow or with the electrical system of the heart are more common after this type of surgery.
Most patients require two to four days in the intensive care unit after repair of a complete atrioventricular septal defect, and a five- to seven-day hospital stay. Recent results indicated that operative survival is around 97 percent for this type of operation.
The most common later problem that is seen following surgery is a leaky mitral valve which may require reoperation in up to 10 percent of patients, but most become medication-free and are free of cardiac symptoms.
Follow-up visits with the cardiologist are important to assess valve and heart muscle function and continued antibiotic prophylaxis for endocarditis is recommended.
Adult and Adolescent Management
Most adult patients with an AVSD have had surgical repair. Most continue to do very well for the rest of their lives, but patients should be monitored by a congenital heart expert periodically because of the potential problems of a leaky or narrow “mitral” valve, subaortic stenosis (obstructed blood flow to the body from the main pumping chamber), high blood pressure in the lungs (pulmonary arterial hypertension), and heart rhythm problems. A small minority of patients will require reoperation as adults because of these problems.
AVSDs occur in so-called “partial” or “complete” forms.
Adult patients with a complete AVSD who have not been repaired almost always have pulmonary arterial hypertension and require careful management. Some patients with a partial AVSD may have the same problem. The complete form of a VSD is most common in patients who have Down syndrome or so-called trisomy 21.
Adult patients with a partial AVSD or so-called ostium premium ASD will usually require surgical repair as adults if not repaired by that time. This is because the right heart chambers enlarge as for any type of atrial septal defect, and eventually problems will occur that will impair patients’ physical abilities and shorten their expected lifespans.
5. Tetralogy of Fallot (TOF)
Tetralogy of Fallot (fah-LO) is a combination of four birth defects that together affect the structure of the heart and how blood flows through it.
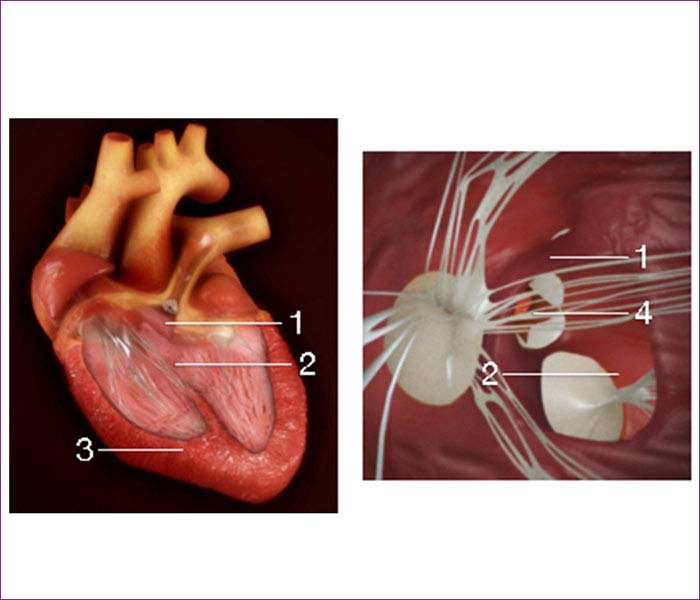
The four defects that together make up tetralogy of Fallot are:
The combined effect of these defects is an inadequate supply of blood to the lungs, which causes blood low in oxygen to circulate to the rest of the body. This lower oxygen level causes cyanosis, which is a blue or purple tint to the skin, lips, and fingernails.
Children with tetralogy of Fallot
A child whose TOF is not repaired might need to limit his or her participation in competitive sports and other physical activities. Many infants who have surgery to correct the defect do very well, participate in normal kid activities, and live to adulthood.
Causes
Science has not yet identified a specific cause for tetralogy of Fallot in all cases, but genetics is believed to play a role. Someone born with TOF seems more likely to have a child with it.
Mothers who contract rubella or other viral illnesses during their pregnancies are at a higher risk of giving birth to babies with TOF. Other risk factors and conditions include poor nutrition, alcohol abuse, diabetes, and mother's age (over 40).
According to the Centers for Disease Control and Prevention (CDC), the presence of certain environmental factors, such as carbon monoxide, might increase a mother's chances of delivering a baby with TOF. In addition, children who have certain genetic disorders, such as Down syndrome and DiGeorge syndrome, often have congenital heart defects, including tetralogy of Fallot.
Signs
One of the most common signs of tetralogy of Fallot is cyanosis (a blue or purple tint to the baby's skin, lips, and fingernails). A child with TOF might experience sudden episodes of cyanosis, called "Tet spells," during crying or feeding.
Other signs include:
Diagnosis
Your doctor may use several diagnostic tests to determine if your child has tetralogy of Fallot, including:
Treatment
Tetralogy of Fallot is repaired through open-heart surgery soon after birth or later in infancy, depending on the baby's health and weight and severity of defects and symptoms. The two surgical options are:
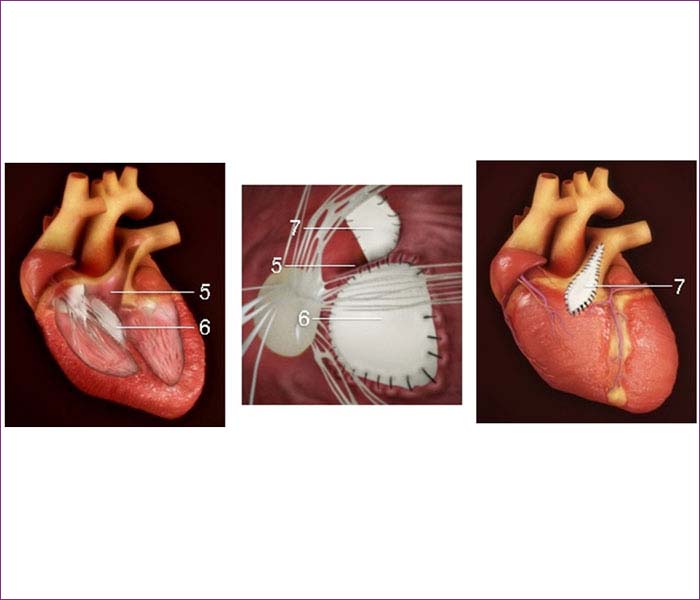
Later when the baby grows stronger, the full repair is performed.
Most babies born with tetralogy of Fallot do very well and survive to adulthood, but require yearly follow-up with a heart specialist.
Total Anomalous Pulmonary VenousConnection Coarctationof Aorta.
TAPVC is a defect in the veins leading from the lungs to the heart.
In TAPVC, the blood does not take the normal route from the lungs to the heart and out to the body. Instead, the veins from the lungs attach to the heart in abnormal positions and this problem means that oxygenated blood enters or leaks into the wrong